The Problem of Enrichment
In the 1970s, the United States developed one of the most powerful technologies you've never heard of — a universal method for pulling apart and isolating atoms at will. Originally designed to enrich uranium for America's nuclear program, it could process metric tons of material before retreating into the confines of a government lab. The underlying physics remained classified, and the system sealed off from the commercial world. Now, half a century later, a startup called Hexium is reviving it, betting that the future of clean energy, materials innovation, and national security might all depend on the forgotten physics of separation.
The periodic table sketches the outline of the atomic world, but not its full texture. Look closer, and you'll find that not all atoms of the same element are truly alike. If elements are a genus, then isotopes are their species. An atom’s element is defined by its protons and electrons, which govern its chemical behavior, but its neutrons, which carry no electrical charge, can shift in number without changing the element itself, giving rise to isotopes.
The most abundant isotopes are perfectly stable. Others, just a few neutrons heavier, become unstable and radioactive. These radioactive isotopes, though chemically similar to their stable siblings, can behave very differently. And it turns out that these unstable variants, scattered across nature, can be exploited for remarkable applications across medicine, energy, dating ancient artifacts, and of course, powering weapons.
Though the promise of isotopes has been known for a long time, isolating them to harness their power has been an agonizing problem for decades. Until fairly recently, we simply didn’t have the kind of precision techniques that could target the exact isotopes we’d need to do such enrichment. This problem became particularly acute during the fifties, when the world’s two major powers, the United States and the Soviet Union, found themselves in a race to produce as many nuclear warheads as fast as possible. The ability to enrich uranium with high concentrations of its fissile isotope, uranium-235, from the naturally abundant and non-fissile uranium-238, was the fundamental rate limiter.
The best solution at the time was gaseous diffusion. Uranium was first reacted with fluoride and converted into a gas, uranium hexafluoride, and thereafter forced through a series of porous membranes. Because uranium-235 is slightly lighter than uranium-238, its atoms moved just a bit faster and diffused through the membranes at a slightly higher rate.
Of course, one stage wasn’t nearly enough, so thousands of these steps had to be chained together to gradually raise the concentration. It worked, but the process was slow, massive in scale, and consumed staggering amounts of electricity. Entire cities' worth of power were needed to keep the machines running. The K-25 diffusion plant in Oak Ridge, Tennessee, for instance, used more than 5% of the entire US electricity supply to power its operations. The Tennessee Valley Authority power network was massively expanded to power these enrichment plants.

Then came the laser. Once thought impossible by the likes of Niels Bohr and even Von Neumann, the laser was a momentous invention, because for the first time ever, we could produce a single, coherent frequency of light. That coherence unlocked a radically different approach to isotope separation. Every element, and even each isotope of the same element, has slightly different energy levels, meaning they absorb and ionize at different, precisely defined frequencies. With a finely tuned laser, you could selectively excite and ionize only the isotope you wanted, plucking it out of a stream of atoms with surgical precision.
Lawrence Livermore National Laboratory, co-founded by Edward Teller in 1952 to accelerate America’s nuclear weapons program, pounced on this opportunity in the early 1970s. Their mission was to develop a laser-based enrichment technique that could outperform the vast, power-hungry diffusion plants of decades prior.
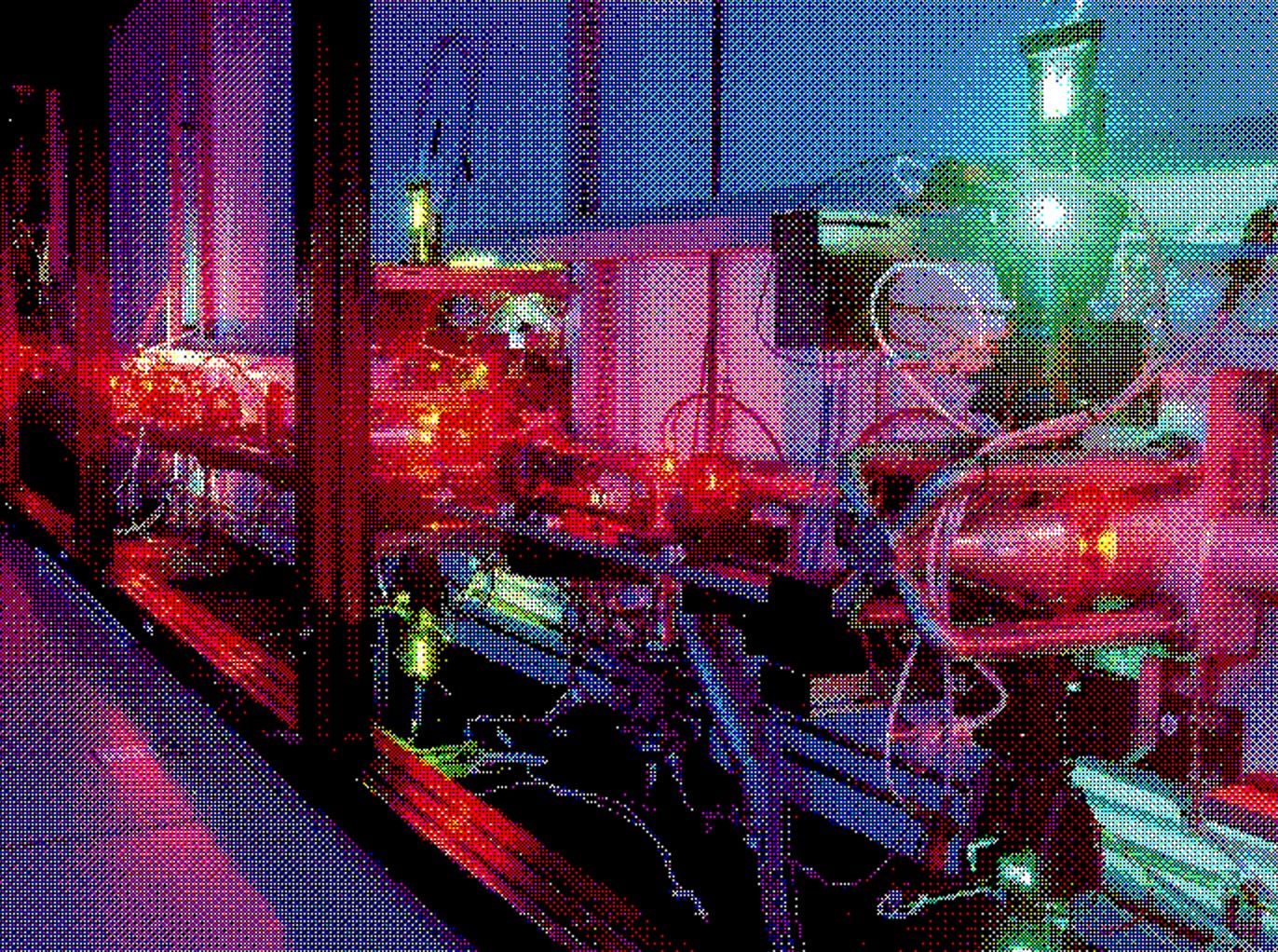
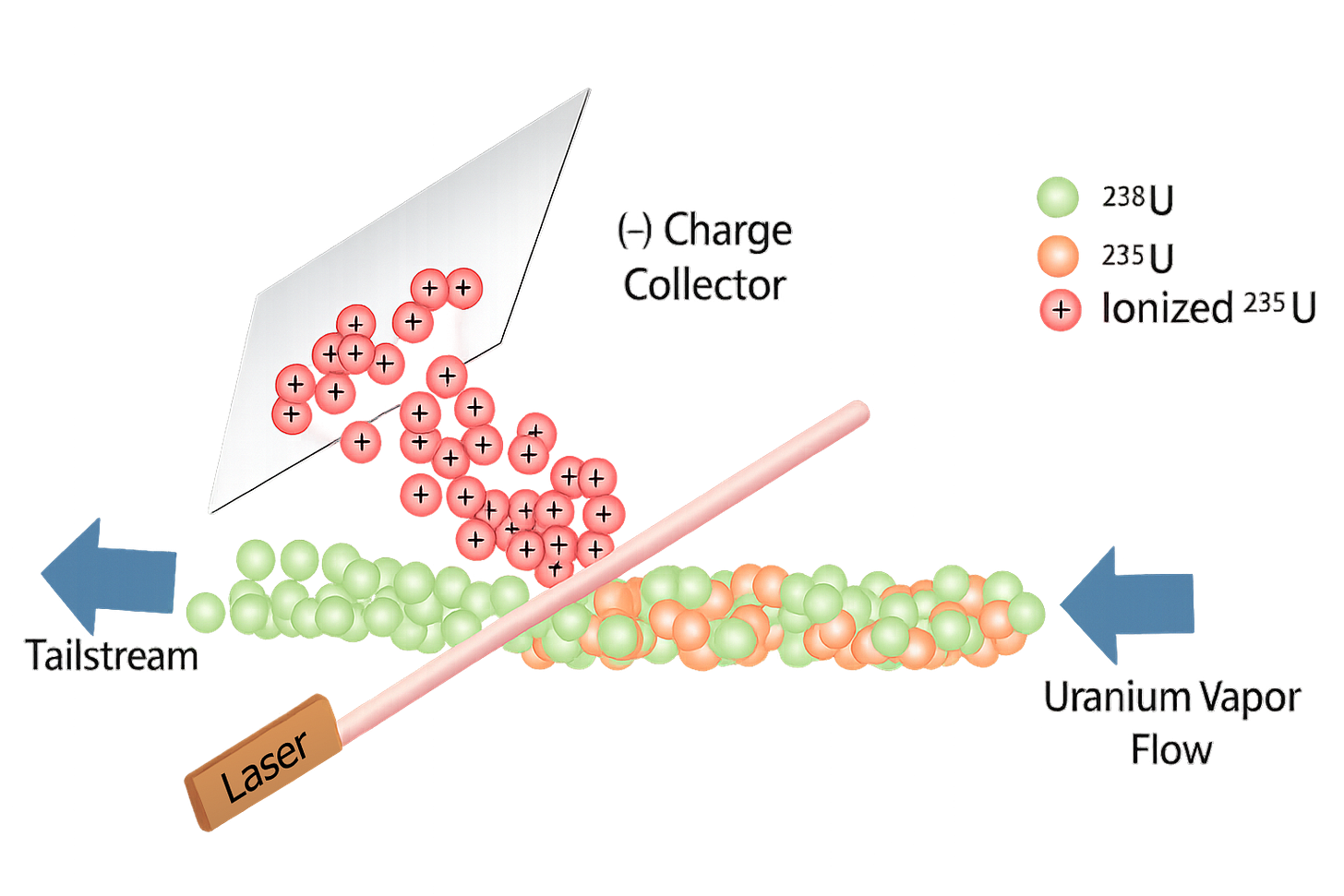
The premise was strikingly elegant; take raw uranium, vaporize it into a stream of individual atoms, and pass a laser tuned to the exact frequency that would ionize only uranium-235. The ionized atoms could then be collected using an electric field, leaving behind the unaltered uranium-238. No pre-processing, and just one-twentieth of the power of a diffusion plant required. The Atomic Vapor Laser Isotope Separation program, or AVLIS, launched in 1973, and just one year later, it was already enriching uranium.
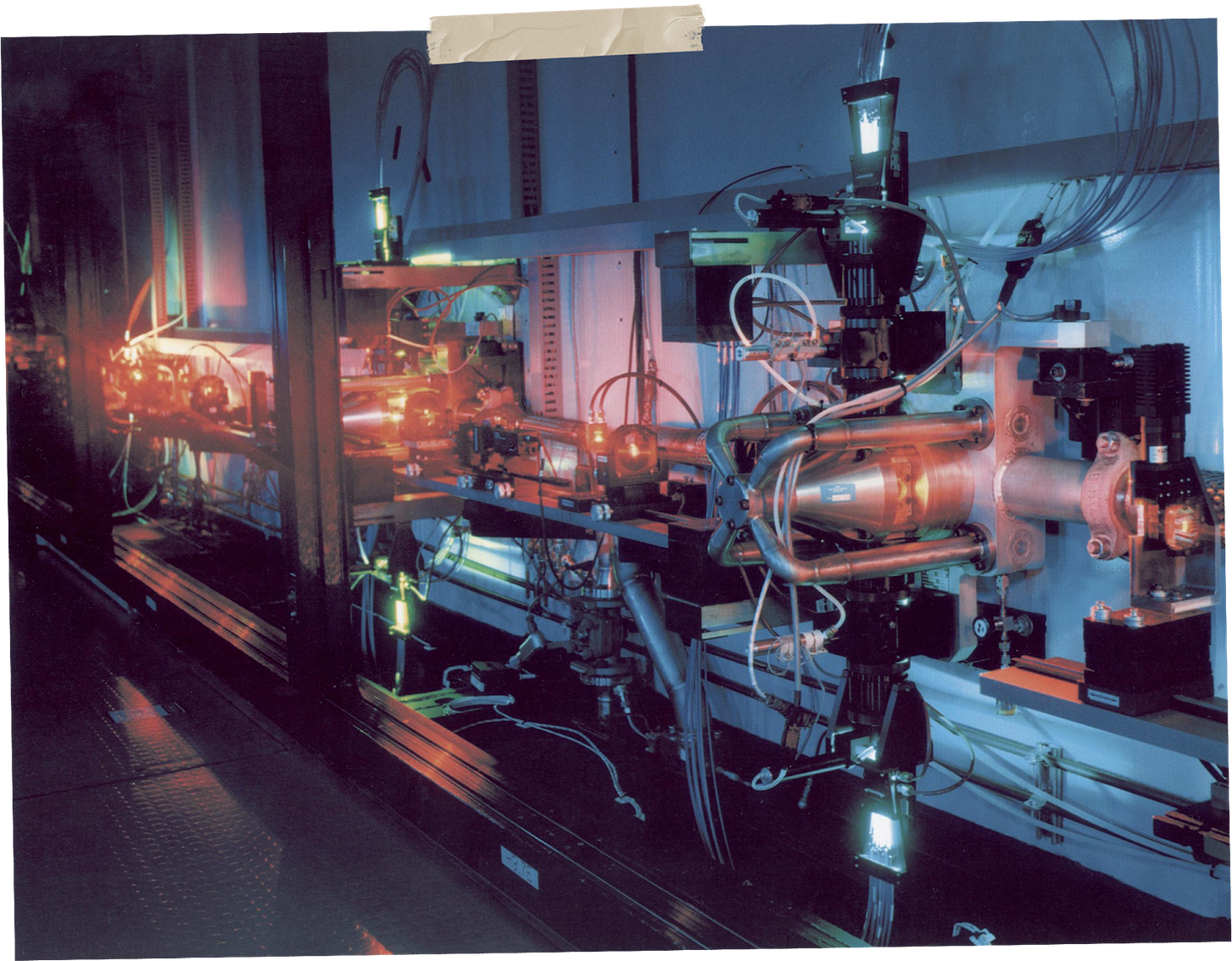
This wasn’t just a technique that could work on uranium. It was a universal platform that, with a few tweaks, could separate any isotopes we might need. It was just a matter of tuning the lasers to the right frequencies. That meant cheap, fast enrichment of fuel for nuclear power plants, a steady domestic supply of isotopes for nuclear medicine, and precision separation techniques that could open entirely new frontiers in materials science.
But just as the lab was preparing to turn a scientific breakthrough into a commercial reality, the world shifted beneath its feet. The Soviet Union collapsed. Nuclear power began to fall out of political favor, and under the terms of a new non-proliferation agreement, the Megatons to Megawatts program, a new cash-strapped Russia began offloading hundreds of tons of its weapons-grade uranium into the US market. Enriched uranium, once a strategic bottleneck, was suddenly abundant and cheap.
The impact of this program was actually quite stunning. Russia’s down-blended uranium ended up powering roughly 10% of all US electricity for twenty years. One in ten lightbulbs in America were running on the remnants of Soviet warheads. However, this also meant that the economic case for AVLIS evaporated. The technology was boxed up, and its potential largely forgotten.
Decades later, Charlie Jarrott found himself inside the very lab where AVLIS had been built. He was part of a new generation, working not on uranium enrichment, but on the next great energy frontier — fusion. However, Lawrence Livermore was not the same organization it was in Teller's day. Speed was no longer the priority it had been at the peak of the Cold War.
Nowhere was this more apparent than at the National Ignition Facility — the largest and most powerful laser system on Earth, designed to achieve fusion by focusing 192 laser beams onto a tiny hydrogen fuel pellet, compressing it with such extreme force and speed that it mimics the conditions at the heart of stars. It was a marvel of engineering, but also a monument to delay. Years of missed milestones earned it the nickname the “Nearly Ignition Facility,” or sometimes the “Never Ignition Facility.” That is, until 2022, more than two decades after construction began, when NIF finally achieved what it set out to do: demonstrate net energy gain from a controlled fusion reaction.
This was a resounding success for the fusion industry as a whole, but as Charlie came to realize, particularly after leaving the lab to work at a fusion startup, cracking ignition wasn’t the only barrier to commercialization.
Fusion’s Glaring Gap
Sustaining a fusion reaction wasn’t just a question of reactor design, whether inertial or magnetic confinement. It also came down to fuel. Two hydrogen isotopes need to fuse together to produce helium and release a powerful burst of energy — deuterium and tritium. Deuterium has one neutron, occurs naturally and can be separated straightforwardly from water. Tritium, with two neutrons, is unstable, radioactive and vanishingly rare. Tritium atoms are produced high in the atmosphere by cosmic rays, in quantities so minuscule they’re completely useless at industrial scale.
To sustain a power plant, the tritium must be bred on-site, created inside the reactor using lithium-6, an isotope that can absorb high-energy neutrons and produce tritium in response. The only problem is that lithium-6 itself is rare and must be enriched, separated from its more abundant cousin, lithium-7.
The United States has no infrastructure to enrich lithium-6. Currently, it relies on stockpiles accumulated during the height of the Cold War. In the 1950s, the most energy efficient way to separate lithium-6 relied on column exchange, or COLEX for short, developed specifically for this purpose. The insight behind COLEX was that lithium-6, by virtue of being slightly lighter than lithium-7, had a slight preference for bonding with mercury.
By running liquid mercury and a lithium-hydroxide solution in opposite directions through tall separation columns, the lithium-6 isotopes gradually migrated out of the hydroxide mix and into the mercury. This was cheaper than alternatives, but extracting the isotopes was toxic. The Y-12 plant, in Oak Ridge, Tennessee, a neighbor of the K-25 gaseous diffusion plant, leached mercury into the soil and nearby waterways. Its operations were ultimately shut down in the sixties, but clean up efforts are still ongoing.

We don’t know the exact size of America’s lithium-6 stockpile accumulated through this process. This fact remains a tightly guarded national secret. But we do know that it is certainly not enough to power and sustain a burgeoning commercial fusion industry.
Around the time NIF achieved its long-awaited demonstration of net energy gain, Charlie met his future co-founder, Jake Peterson. They were both preparing to leave the fusion startup they had been working at, each for different reasons. When the two connected to discuss the state of the fusion industry, they came upon a troubling realization. There were already some 25 private fusion startups in the United States, but no domestic source for enriched lithium-6.
For Charlie, the solution was hiding in plain sight. He’d seen it first-hand at Lawrence Livermore. The AVLIS program which once promised universal isotope separation needed to make a comeback, and if it could work for uranium, it could certainly be made to work for lithium. In fact, it would be easier. Uranium had to be heated to 2,000 degrees Celsius and vaporized into a plasma to run the process. Lithium melted at just 180 degrees. You could do it in your oven. And unlike uranium-235, which makes up less than one percent of natural uranium, lithium-6 occurs at concentrations nearly ten times higher, making the starting material far richer from the outset.
Laser technologies, which were still nascent in the seventies, had also undergone a transformation so profound it bordered on the miraculous. What was once experimental, from narrow-linewidth beams, to stable frequencies and high repetition rates, was now commonplace and commoditized. Lasers were playing a role in nearly every domain of modern life, from semiconductor fabs to digital communications, manufacturing to medical technologies and more. The entire system could be built with off-the-shelf laser technology and use computerized feedback loops that would make constant manual tuning of lasers a relic of the past.
Everything could be miniaturized, and built modularly. What once required a sprawling government facility could now fit inside a single 20-foot container. All you needed was a centimeter-wide slit to admit a vapor stream, and a set of three tuned lasers pulsing at just the right frequencies to selectively ionize your isotopes. From there, standard electric fields could separate the ions from the stream.
The physics were proven. The materials were simpler. The economics were so good, they were almost unbelievable. The going rate for a kilogram of enriched lithium-7 was $10,000. For lithium-6, it was at least ten times that. Meanwhile, natural lithium could be bought for just $30 a kilo.
Charlie and Jake looked at the problem and saw inevitability. The market need could be proven deterministically, and more importantly, it was needed yesterday. Could one imagine a more opportune moment to revive AVLIS?
They incorporated their new company under the name Hexium. The Greek prefix hex-, a nod at their isotope of interest.
Beyond Fusion
Then — they hit a wall. Investors simply didn’t believe that lithium-6 was something anybody would want or pay for. All the fusion companies were already funded, and none of their budgets had line items for lithium-6. Many were operating on the premise that a natural lithium blanket inside the reactor would be sufficient to breed the tritium they would need, and their backers were convinced. Beyond that, no one really wanted to fund a company whose main customer would be other fusion companies, most of which were years away from operating reactors, let alone generating recurring, predictable demand.
Finally, in May of 2024, Hexium received their first few checks from Humba, followed shortly thereafter by Julian Capital. As their early backers learned more about the technology, they realized that convincing follow-on investors around the urgent market need might just require modifying the pitch. After all — by anchoring the company’s story on solving fusion’s hair-on-fire problem with lithium-6, Hexium was overlooking a whole other product line they had access to for free. One which could hit the market far sooner than fusion. Lithium-7.
Lithium-7, a byproduct of enriching lithium-6, is a critical component of molten salt reactors, or MSRs, an advanced class of fission reactors gaining traction in the US. These reactors use molten fluoride salts, often containing lithium, to dissolve fuel and transfer heat. This design promises higher efficiency, passive safety, and operation at atmospheric pressure. But it also demands extreme chemical purity. The reasons that make lithium-6 great for fusion are the same reasons that make it a nightmare in molten salt reactors. Too much of it, and the system will flood with radioactive tritium, increasing the amount of radioactive waste and creating unnecessary engineering challenges around tritium capture and containment. To contain these side effects, molten salt reactors need high-purity lithium-7 — but the only players in the world that can supply it are China and Russia, both of which use COLEX.
With companies like Kairos Power and Natura Resources now building MSRs with federal backing, demand for lithium-7 is set to rise. The Department of Energy is investing billions into advanced reactors, and even modest deployment could create a $1–3 billion annual market for lithium-7. And unlike fusion, which is still chasing ignition, these fission reactors are already being built.

“Anytime we interface with a fission company, they want us to pivot and leave fusion behind completely,” says cofounder Jake Peterson. “With the scientific discovery largely behind them, they understand the critical need to develop their supply chains immediately, as they focus on building toward commercial deployment and revenue generation.”
It wasn’t just the engineers who were excited. Investors, too, began to lean in once they realized that Hexium wasn’t just building a fuel source for some distant fusion dream, it was unlocking an immediate market in advanced fission too.
Ironically, nailing the pitch was one of the most trying challenges for Hexium in its early days, a seemingly small issue for a technology with such profound capabilities. But once Hexium helped investors connect the dots, the tide turned. Charlie and Jake ended up raising $12 million across two early, oversubscribed rounds.
From there, momentum built quickly. The team had a clear sense of purpose, not to replicate the old system, but to build something entirely new, guided by their CTO, Martin Griswold, a fellow fusion veteran from TAE. At Lawrence Livermore, where the original AVLIS program was born, researchers who had quietly carried the weight of its unrealized promise were thrilled to see a team finally trying to bring the underlying vision to life. Many offered to help in any way they legally could.
Charlie is now in the process of reactivating his Q-clearance, the same high-level classification once granted, and famously revoked, from J. Robert Oppenheimer. While Hexium’s design is entirely its own, the clearance may one day allow Livermore to provide technical feedback, insights that could shave years off development. After all, the lab had once built a pilot system capable of separating uranium at metric-ton scales, a far more complex challenge than lithium. After sitting dormant in classified archives, sealed off from industry and public use, AVLIS now has a path back to the world.
Making Picks & Amps
When asked about the tech itself, Charlie and Jake describe it very modestly. “It’s just three metal boxes on a table,” Charlie says. “It looks like a workstation or a high-performance PC, nothing exotic.”
Sadly, you can’t actually see the lasers in operation as they ionize the atoms. “They’re class-four lasers,” Charlie says. “Powerful enough to cause permanent eye damage in an instant, even from a reflection.”
“It looks a little unremarkable,” Jake adds. What’s happening inside those boxes, however, is anything but. At the heart of the system is a process that is the closest we’ve ever come to manipulating individual atoms at industrial scale.
And yet, making it feel unremarkable is precisely the point. “Our job is to make this simple,” Jake says. “We don’t want people worrying about exotic chemistry or some opaque process involving crown ethers or toxic reagents. It should feel boring.”
“Boring and predictable are good things in a supply chain,” Charlie adds. “Especially when your core business is already full of scientific and engineering risk.”
At a time when the spotlight is fixed on technologists pushing the boundaries of physics, whether by standing up fusion reactors, reshaping the energy grid with advanced fission, building quantum computers, or bringing a spacefaring civilization within reach, someone still has to create the infrastructure that makes it all possible. In a world where everyone wants to be a rockstar, Hexium is content making the picks and amps. “That’s what we’re building,” Jake says. “The infrastructure that enables everything else.”
The beauty of Hexium’s system lies in its composability. Each unit is lightweight and configurable, designed to operate independently or as part of a scalable, parallelized system. That means Hexium can rapidly spin up capacity to meet demand, but also tailor facilities to customer-specific requirements.
One section of a plant can enrich to a specific level, the next can target a different isotope entirely, and all of it can operate on shared infrastructure. This composability allows Hexium to navigate both market uncertainty and technical variation, adapting in real time to different isotopes, enrichment levels, and customer needs. It’s a new kind of hardware architecture, built to be reconfigurable and built to evolve.
Though this composability raises a provocative question — in a world where fusion works, does every reactor get a Hexium system next door?
It’s a tempting idea, a decentralized network of compact isotope separation units, feeding enriched lithium-6 directly into nearby fusion plants. But that vision quickly runs into a tangle of operational and geopolitical complexity. For starters, enriching lithium-6 at scale doesn’t just produce your desired kilograms of fuel, it also generates thousands of kilograms of lithium-7 as a byproduct. A valuable resource for fission, yes, but a logistical headache for fusion companies that have no use for it. It’s not something they want to store, let alone ship.
Hexium is more than happy to find a buyer for the lithium-7. But there’s another, more sensitive issue lurking beneath the surface — proliferation. The AVLIS method, the core of Hexium’s technology, was originally developed to enrich uranium. It’s not just efficient. It’s too efficient. The kind of thing that makes governments nervous. That’s why it requires Q clearance to operate, and also why it lives under lock and key at national labs.
“The problem with any revolutionary enrichment technology like AVLIS is that it simultaneously enables critical breakthroughs and creates real proliferation risk,” Charlie tells me. “Scattering these systems across the country complicates the kind of centralized oversight needed to prevent misuse."
The concern isn’t hypothetical. With enough decentralized systems, you open the door to covert uranium enrichment, by rogue states, bad actors, or anyone with the ambition and means to quietly repurpose a laser. The defense and reconnaissance community prefers centrifuges for a reason. “Centrifuge enrichment facilities are large-scale industrial systems,” Charlie explains. “They draw substantial power, demand purpose-built infrastructure, and leave a footprint that’s clearly visible from space.”

Hexium’s system doesn’t draw much power. It doesn’t need a giant footprint. It fits inside an ordinary building. That’s precisely what makes it so powerful, and so sensitive.
In the end, operations may settle on a centralized model. One facility serving many customers. “If fusion succeeds,” Charlie says, “we can be the ExxonMobil of the ecosystem, quietly supplying the fuel that powers it all.”
The Dow Chemical of Isotopes
Lithium isn’t actually Hexium’s end goal. It’s the starting point. As they scale, Charlie and Jake aren’t just thinking about energy. They’re thinking about matter. They're thinking about isotopes the way chemists once thought about elements, as the base units of invention. Their long-term ambition is to become something like the Dow Chemical of isotopes, a supplier of atomic building blocks for entirely new industries.
There are dozens of isotopes, hundreds, even, that are bottlenecked by production.
“Iron has four isotopes,” Charlie points out. “Some have high neutron capture cross sections and become radioactive. Others are effectively transparent to neutrons. That distinction matters when you’re engineering structural materials for sustained neutron exposure and considering radiation hardening requirements.”
However, if you can isolate the neutron-transparent isotopes, you can actually make neutron-hard materials. This isn’t only valuable for nuclear plants. Consider the distant future when technologies like fission or fusion-powered propulsion systems may be powering the spacecraft taking humanity to distant, cosmic shores. Shielding around these reactors will be critical to protecting the craft’s vital systems and ensuring the safety of human crew.
And the possibilities go far beyond structural metals. There’s silicon-28, already a cornerstone of silicon-based quantum computing. Or the copper isotopes that are reshaping nuclear medicine. Copper-64, used in PET imaging, helps doctors detect and monitor cancers at the molecular level. Copper-67 delivers targeted radiation directly to tumor cells with minimal damage to surrounding tissue. “The space for new applications is far from saturated,” Jake says. “We’re still at the front end of what isotopes can unlock.”
Building a fully universal isotope separator will require new tools. Right now, Hexium uses state of the art, solid-state, narrow bandwidth lasers, purposefully tuned to the exact energy levels needed to isolate lithium’s isotopes. But new innovations in laser technology are on the horizon. Optical parametric oscillators, for instance, can shift frequency with a simple rotation of the crystal, allowing a single system to dynamically target multiple isotopes. No hardware swaps. No rebuilding. Just tuning the dial and pointing the beam.
That’s still a few years out. For now, Hexium is focusing first on lithium, then on scaling. But they’re building with the long arc in mind. Because if fusion works, if quantum computing works, if interplanetary propulsion works, somebody has to build the atomic supply chain. That’s the vision they’re building towards. “We want to unlock the frontier for others,” Jake says. “No one should be held back because the right isotope doesn’t exist at scale.”